Method Validation Edition
Validate and verify your analytical and diagnostic methods to meet the demands of regulatory compliance.
Method Validation Edition
Validate and verify your analytical and diagnostic methods to meet the demands of regulatory compliance.

The leading software package for method validation for over 30-years.
Analyse-it is developed for and is in use at thousands of ISO/IEC 17025 accredited testing and calibration laboratories, ISO 15189 accredited medical laboratories, CLIA '88 regulated medical laboratories, and IVD manufacturers for development, support, product labeling and FDA 510(k) submissions.
Built for CLSI protocols
The latest Clinical and Laboratory Standards Institute (CLSI) method validation protocols are recognized by the College of American Pathologists (CAP), The Joint Commission, and the US Food and Drug Administration (FDA).
That's why we included extensive support for 11 CLSI protocols

|
EP05-A3 Evaluation of Precision of Quantitative Measurement Procedures |
EP15-A3 User Verification of Precision and Estimation of Bias |
|
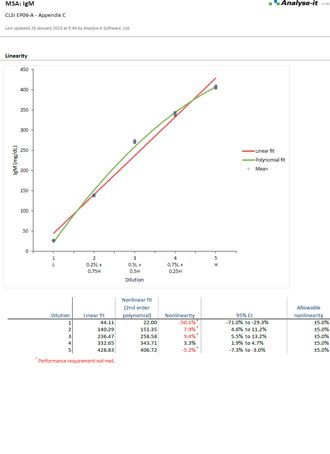
EP06-A Evaluation of the Linearity of Quantitative Measurement Procedures |
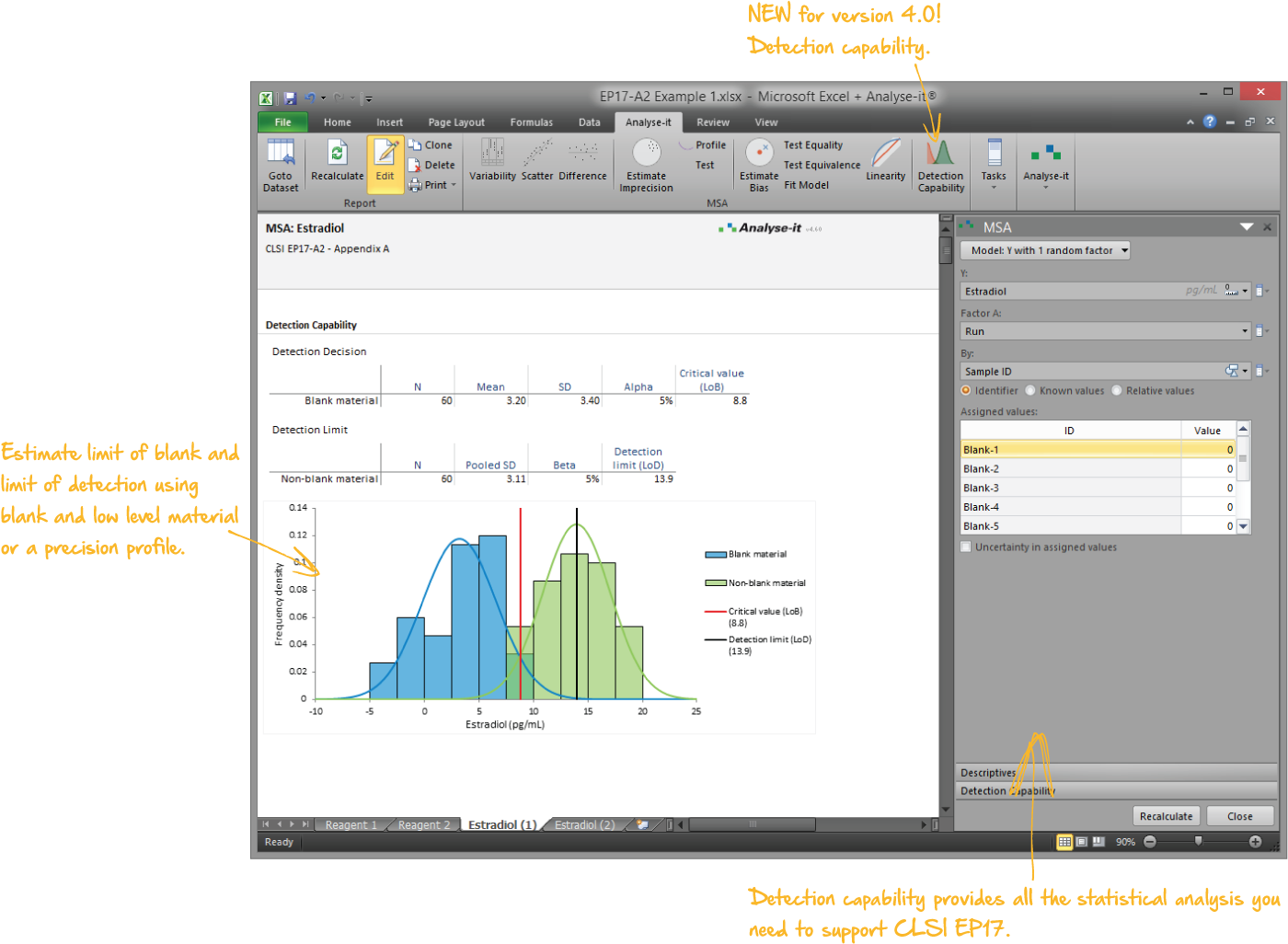
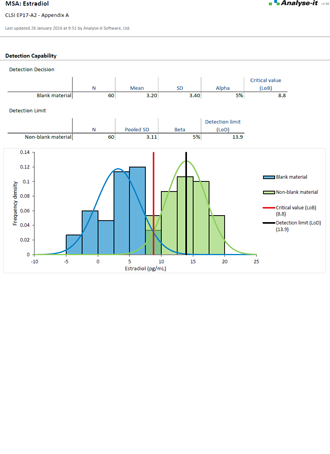
EP17-A2 Evaluation of Detection Capability for Clinical Laboratory Measurement Procedures |
|
EP09-A3 Measurement Procedure Comparison and Bias Estimation Using Patient Samples |
EP21-A Estimation of Total Analytical Error for Clinical Laboratory Methods |
|
EP10-A3-AMD Preliminary Evaluation of Quantitative Clinical Laboratory Measurement Procedures |
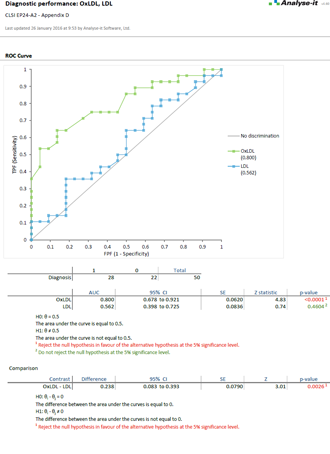
EP24-A2 (Replaces GP10-A) Assessment of the Diagnostic Accuracy of Laboratory Tests Using Receiver Operating Characteristic Curves |
|
EP12-A2 User Protocol for Evaluation of Qualitative Test Performance |
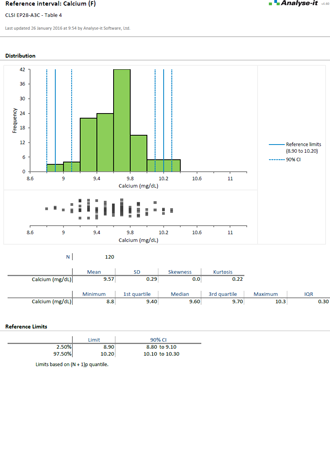
EP28-A3C (Formerly C28-A3C) Defining, Establishing, and Verifying Reference Intervals in the Clinical Laboratory |
|
EP14-A3 Evaluation of Commutability of Processed Samples |
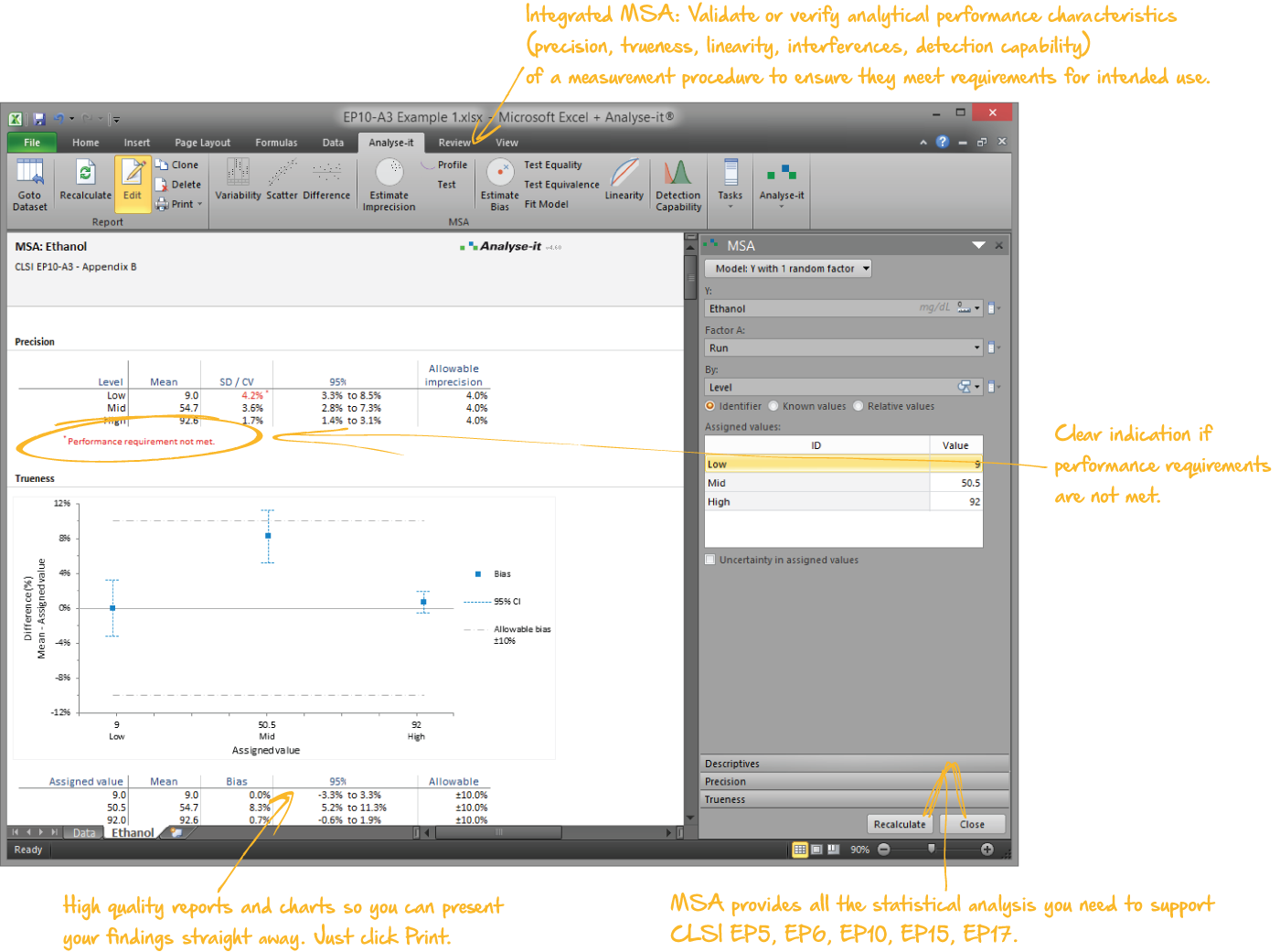
Validate and verify measurement system performance characteristics
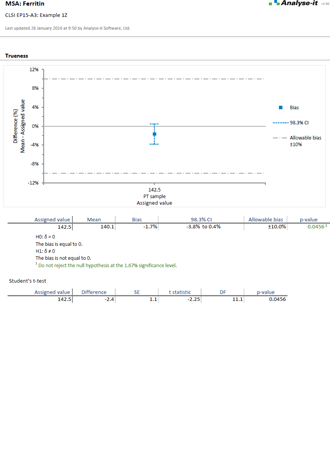
It’s essential to ensure the performance characteristics (precision, trueness, linearity, interferences, detection capability) of a measurement procedure meet the requirements for intended use. Manufacturers (IVD companies) must establish performance during product development to feedback into the development process, for FDA 510k submissions and product marketing, and to support customers in the field. Laboratories must verify they can achieve the manufacturer's claimed performance during implementation of a new measurement system, during regulatory inspections (under the CLIA ’88 act), and as part of proficiency testing (PT) schemes. Measurement systems analysis (MSA) lets you determine all these important performance characteristics in one analysis.
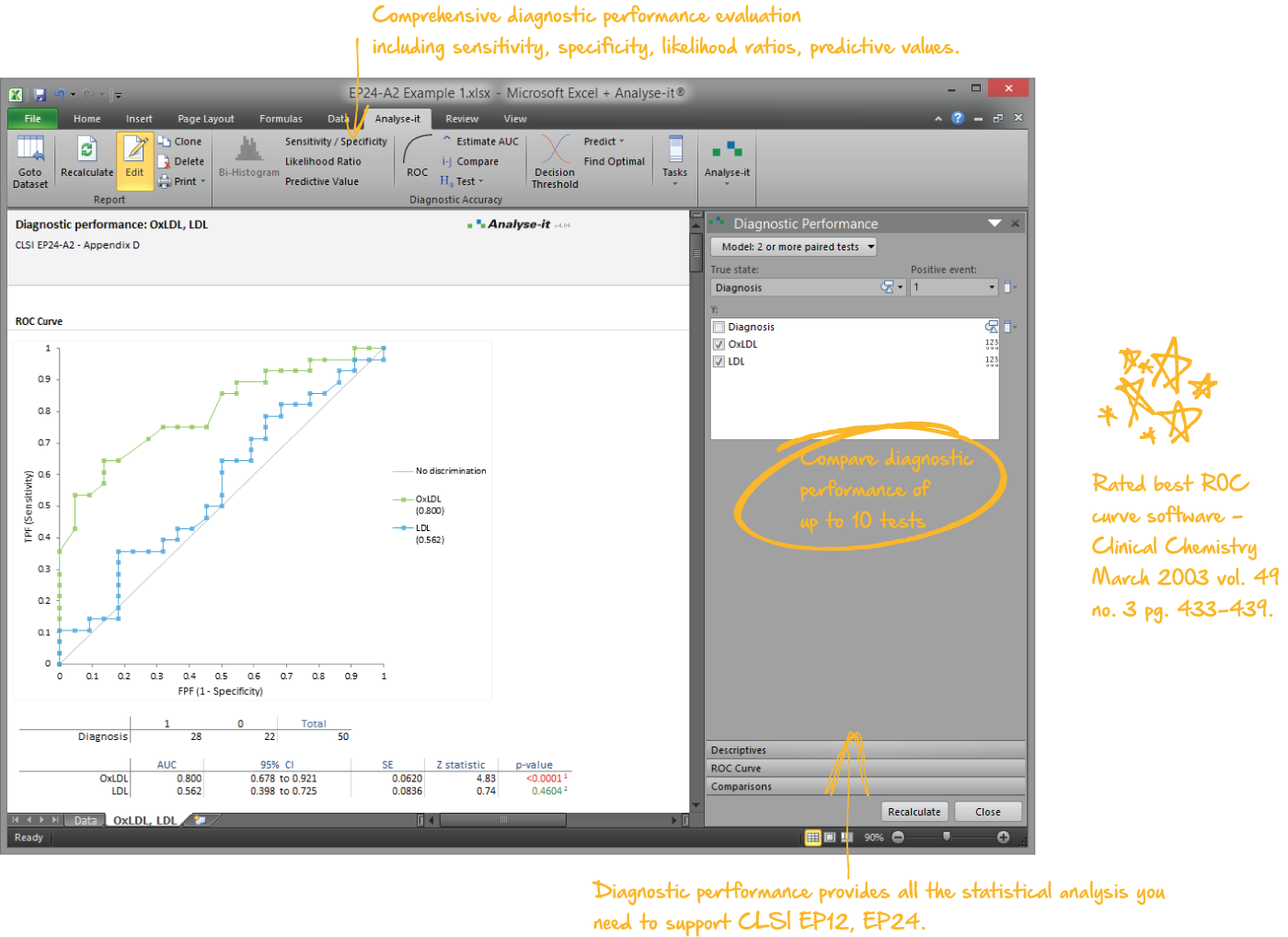
Examine diagnostic test performance to find the most effective
Rated best ROC curve software in Clinical Chemistry March 2003 vol. 49 no. 3 pg. 433-439, Analyse-it lets you establish and compare the ability of a diagnostic test to correctly diagnose patients. Explore how the test differentiates between positive and negative cases and explore optimum decision thresholds factoring in the costs of misdiagnosis.
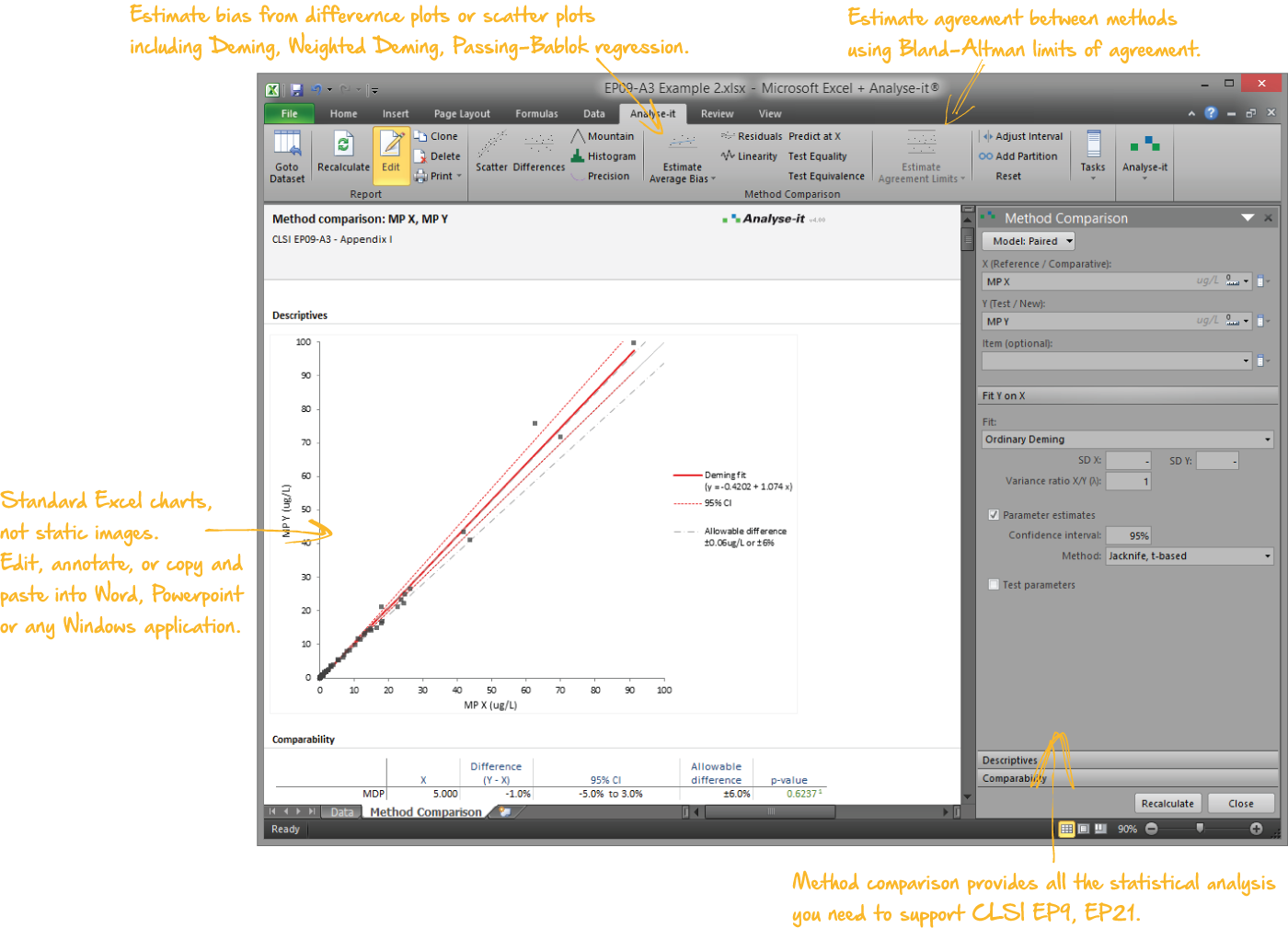
Compare methods and evaluate the impact of making changes
When introducing a new measurement procedure you want to see how it stacks-up against your existing procedure or evaluate its performance against the gold-standard. Bland-Altman lets you see the agreement between methods and what effect the differences between methods might have on clinical interpretation. More advanced procedures like Deming regression and Passing-Bablok tell you the bias between methods, how medical decision points may be affected, and let you test if bias meets performance requirements.
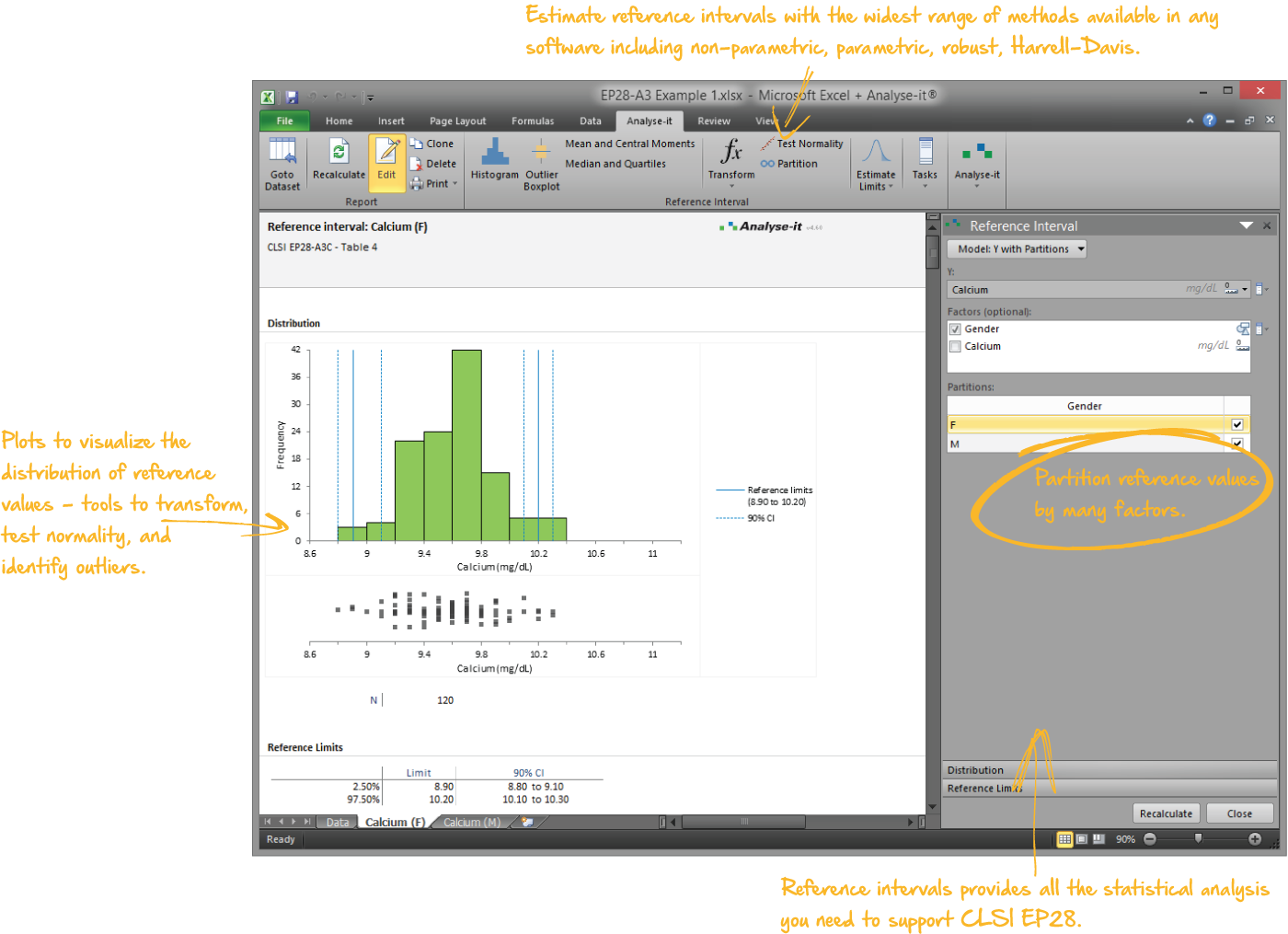
Establish reference intervals to make clinical diagnoses
Reference intervals are essential for clinicians to interpret results and make a diagnosis. As a laboratory it's your job to provide normal reference ranges they can rely on. With the widest range of methods available in any software package, the ability to partition the intervals by factors such as sex, age, ethnicity, Analyse-it makes it easy to establish reference ranges or transfer them to a new measurement procedure.
Plus it includes the
 Standard Edition...
Standard Edition...
All the features from the Analyse-it Standard Edition are included so you can improve processes and products by identifying solutions using hypothesis tests and model fitting techniques.
Integrated into Microsoft Excel, so it's easy to use...
That's right. Analyse-it integrates into Microsoft Excel 2007, 2010, 2013, 2016, 2019, 2021, 2024 and Microsoft 365 for Microsoft Windows. There's virtually no learning curve, and the intuitive user interface and logical task-based workflow makes sense to those of us that aren’t programmers or full-time statisticians.
All your data and results are kept in Excel workbooks, making it easy to collaborate and share them with colleagues, and meaning there's no locked-in file format.

Software you can trust
We've developed software for method validation for more than 30 years, and popularized statistical procedures that have since been adopted into method validation standards and guidelines.
 But it's our customers that matter - the independent laboratories, regulatory agencies, and many of the world's top ten IVD companies. Check out the latest reviews on Capterra, in the meantime here are a few of our customers...
But it's our customers that matter - the independent laboratories, regulatory agencies, and many of the world's top ten IVD companies. Check out the latest reviews on Capterra, in the meantime here are a few of our customers...










Analyse-it® Reduces Method Validation steps at National Reference Laboratory
With Analyse-it, I pull in the data and quickly analyse it, and then prepare figures for manuscripts right there. From the beginning of the project to completion, using one application saves me probably a day's worth of time.
Analyse-it® Cuts Project Time in Half at Swiss Laboratory
Analyse-it has a tremendous advantage in its ease of use. With other programs, you really have to study how to use them, but Analyse-it makes it so easy, and at the same time offers the advanced procedures we need like Weighted Deming regression.
Accurate and reliable
You might have heard Microsoft Excel isn't up to the job of statistical analysis. It isn't. The built-in functions just aren't built for accuracy. So we don't use a single one. Instead, Analyse-it handles all of the calculations internally, using reliable algorithms and IEEE 754 double floating point precision. And the results to prove it?
Validated, tested at every stage
We've conducted thousands of tests to put Analyse-it through its paces. They cover all releases and service packs of Microsoft Excel 2007, 2010, 2013, 2016, 2019, 2021, 2024 and Microsoft 365 for Microsoft Windows. What's more, validation tests are run automatically after every change to the software so you can be confident the statistics are correct. And stay correct.
Dependable support
No need to resort to old textbooks. Analyse-it includes detailed help that’s written in plain English. And if you get stuck we’re here for extra help - aiming to answer your queries within 24 hours.
Take a look for yourself. Real-world example analyses using Analyse-it...
Method comparison
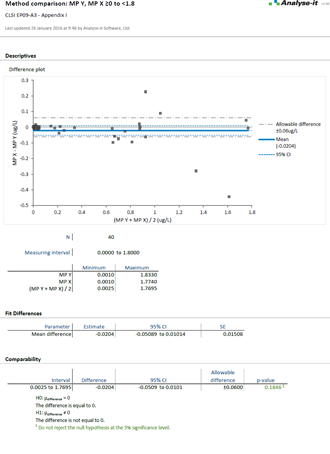
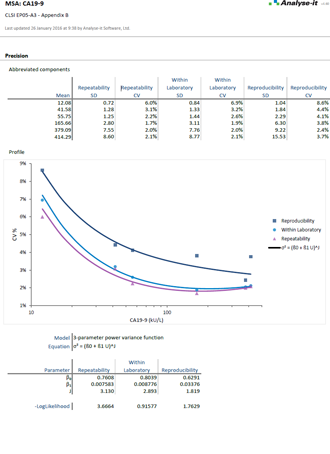
Precision
Included from the Analyse-it Standard edition...
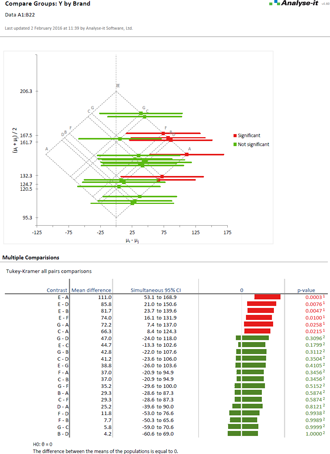
Compare
Technical specification
System requirements |
|